Abstract
Chimeric antigen receptor T (CAR T)-cells targeting CD19 have become one of the most promising therapeutics to combat B cell malignancies. However, toxicity including cytokine-release syndrome (CRS) and neurotoxicity remains unpredictable thus limiting wider application. Soon after the clinical success of autologous CAR T, the development of allogeneic CAR T treatment had begun, taking advantage of lower manufacturing costs and timely delivery of CAR T products. Even within this approach, the same safety concern applies. Particularly, T cells from healthy donors used to produce allogeneic CAR T may carry individual-specific immune activation characteristics once they interact with cancer cells and patient immune systems, and often this is not manifested when tested in vitro or in vivo including non-human primates. This additional factor adds difficulties in predicting individual variability in toxicity and efficacy of CAR T therapy.
To access individual variability in toxicity and efficacy of autologous and allogeneic CAR T therapy in vivo, we have developed a model using NSG™-MHC Class I/II double knock-out (DKO) mice humanized with human peripheral blood mononuclear cells (PBMCs). NSG™ mice lack murine T, B, NK, and functional dendric cells and are optimal to adopt the human immune system. Additionally, MHC Class I/II DKO confers delayed onset of GvHD and low baseline cytokine release when human PBMCs are engrafted. This positions the PBMC-humanized DKO mice as an optimal mouse model to access cytokine induction from CAR T treatment.
Irradiated female NSG™-DKO mice were reconstituted with human PBMCs. CD19 CAR containing CD28 costimulatory domain, CD3z chain, and the protein marker FLAG-tag (modified protein DYKDDDK for tagging), was transduced into two human PBMCs (Donor A and Donor B) and injected into the humanized mice via tail vein. We monitored body weight change and CRS scores throughout the experiments. The levels of human CD45+ (humanization), human CD19+ (efficacy), and FLAG+ (CAR T expansion) cells in blood and spleen were confirmed by flow cytometry.
Dramatic body weight loss and low survival rate were shown in mice humanized with 17M PBMCs from Donor A then received by autologous CAR T (N=12) compared to PBS controls (N=13). The serum levels of IFN-ꝩ, IL-2, IL-4, IL-6, IL-10, TNFα, MIG, MIP-1a, and IP-10 were significantly increased in response to CAR T treatment. Next, we humanized mice with 15M PBMCs of two different human donors (N=12/donor), and treated with autologous CAR T, activated T cells, or PBS. We observed donor variability in toxicity; mice humanized with 15M PBMCs of the same Donor A showed a similar toxicity response, measured by significant body weight loss, but milder than the toxicity observed from mice humanized with the 17M PBMCs. Mice humanized with Donor B PBMCs did not differ in toxicity responses across treatment groups. In both Donor A and Donor B humanized mice, CAR T successfully eradicated human CD19+ cells in peripheral blood and spleen.
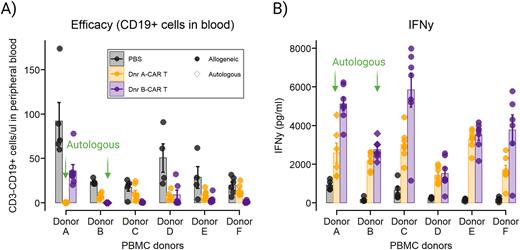
Then we humanized DKO mice with 10M PBMCs from six human donors (N=19-20/donor) and treated them with PBS or allogeneic CAR Ts from either Donor A or Donor B. In the mice humanized with one of the six donors, we observed significant body weight loss only in the Donor B CAR T group compared to PBS control and Donor A CAR T treated. The other five donors did not show significant body weight loss from allogeneic CAR T treatment. Donor B CART showed greater allogeneic efficacy than Donor A CAR T. In both allogeneic treatments, PBMC donor-specific variability was observed. Human cytokine levels showed a distinct cytokine induction dependent on each allogeneic CAR T treatment as well as PBMC donors. Principal component analysis with the cytokine data revealed that Donor B CAR T induced more distinguished cytokine responses than Donor A CAR T. Donor B CAR T induced higher IFN-ꝩ, IL-10, and IL-3 compared to Donor A CAR T, and vice versa was shown for IL-2, IL-5, and IL-9.
In summary, we show that a novel in vivo PBMC humanized mouse model can assess the toxicity and efficacy of both autologous and allogeneic CD19-CAR T treatment. This model provides a platform to pre-screen cytokine induction profiles dependent on the interaction between host and healthy T-cell donor for allogeneic CAR T therapy.
Disclosures
Gustafson:Vifor Pharma: Consultancy. Gardner:Novartis: Honoraria; Crispr Therapeutics: Honoraria; Juno Therapeutics: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal